Redica Systems is a unique data analytics platform that tracks life sciences inspection and enforcement action data, providing an overview of the trends to pharma and medical device companies. They’ve pioneered a new category called Quality and Regulatory Intelligence (QRI). Redica Systems decided to partner with GoodData to get solid data analytics and visualization capabilities. Before Redica Systems, most life sciences companies manually processed all the data separately. Redica Systems’s goal is to save time and increase efficiency in reducing compliance risk.
DOCUMENTS STORED IN REDICA SYSTEMS
62k
INSPECTION RECORDS STORED IN REDICA SYSTEMS
900k
INTEGRATED INTO ITS PRODUCT PORTFOLIO
50 GoodData workspaces
PERCENTAGE OF TOP PHARMA AND MEDTECH COMPANIES THAT USE REDICA SYSTEMS
90%
The company
Redica Systems, a Quality and Regulatory Intelligence (QRI) platform for pharma and medtech companies, transforms millions of data points into meaningful answers and insights, improving product quality and reducing regulatory risk.
As a life sciences data analytics company, Redica Systems ingests data from the world's various regulatory and inspection agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Redica Systems gathers and sorts third-party data, analyzes trends, and provides its customers (specifically pharmaceutical and medical device companies) with detailed analysis. Redica Systems makes the analytics easily accessible for its clients, providing the foundation for better decision-making.

Data about past inspections, internal audits, new standards, and regulations fall into Redica Systems’ three categories of focus: inspection preparation, vendor quality monitoring, and regulatory surveillance. These areas produce a huge amount of unstructured data that is difficult to comprehend without proper analysis.
To make efficient decisions based on risk level, it is in the interest of life sciences companies to know about the latest agency inspection focus and any recent enforcement actions. In the past, many pharma and medtech companies tried to analyze the data manually, but the process was slow, laborious, and failed to produce the desired outcomes.
This is where Redica Systems comes in. Their goal is to filter out the noise and surface insights that improve decision-making. By turning unstructured and siloed data into easily accessible, user-friendly analysis, they strive to save time and increase efficiency for life sciences professionals.
Redica Systems began to monitor the trends and set about building the world's first platform dedicated to Quality and Regulatory Intelligence (QRI). They track inspections, enforcement actions, and regulatory publications, analyze the data, and provide pharmaceutical and medtech companies with an overview of any regulatory risks associated with their global supply chains.
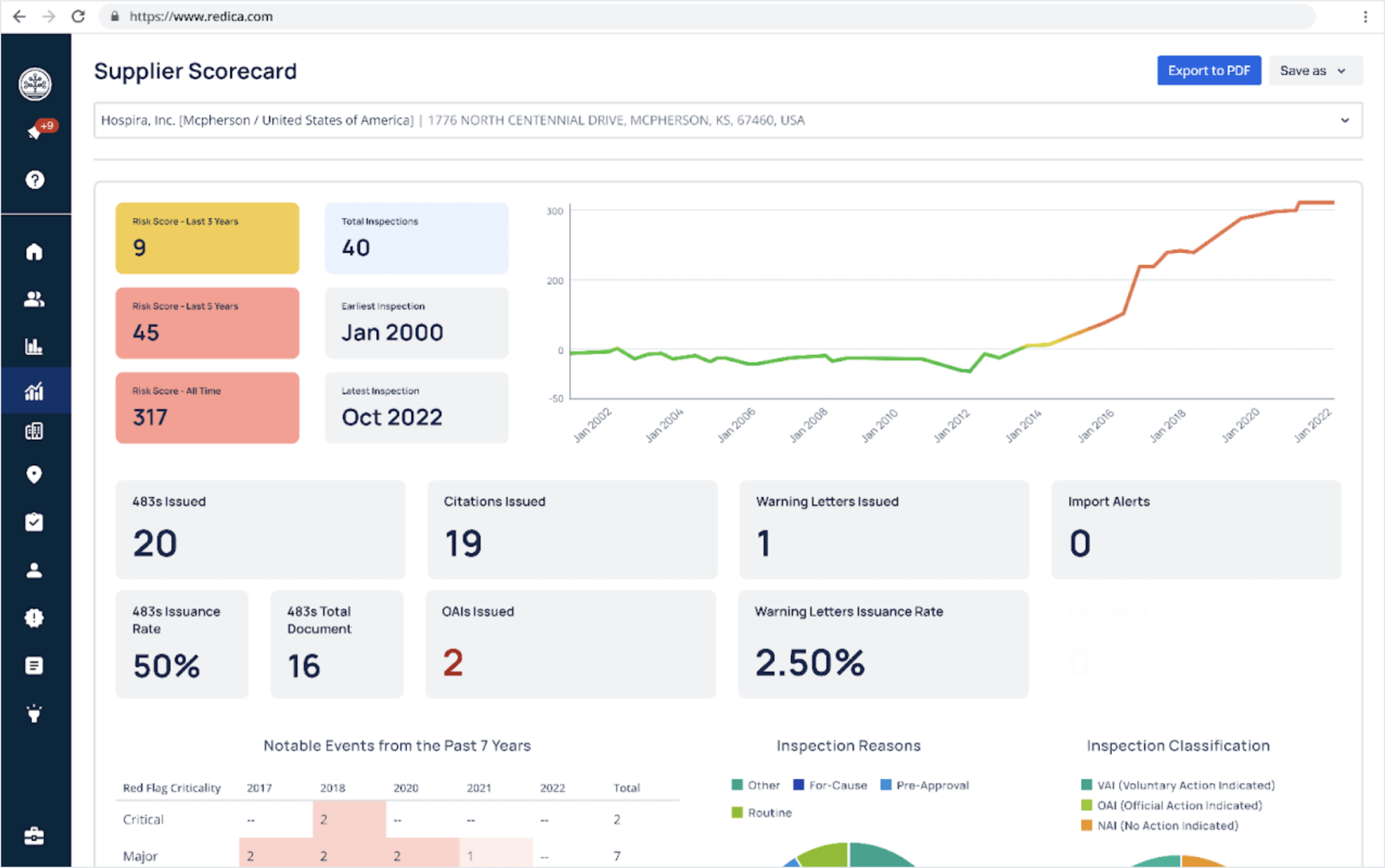
The core of the platform is dashboards and data visualizations. These are integrated into the application and monitor key proprietary metrics, like Supplier Risk Score™. Redica Systems uses 50 GoodData workspaces to provide analytics, each one tailored to the specific client's use case.
Top GoodData features that match Redica's needs
Embedding via React SDK
undefined
GD's React SDK embedding method was chosen for its ease of interactivity and customization.
Time to market
undefined
Implementation time was 50% shorter with GD than with other traditional BI tools.
Semantic layer
undefined
GD’s semantic layer and logical data model allow Redica to process large amounts of data and generate real-time views.
The result
With its life sciences QRI, Redica Systems managed to turn the unstructured data into easily accessible and user-friendly analytics that are used by over 200 leading pharmaceutical and medical device companies. Customers gained access to a large volume of enriched data, most of which was inaccessible before. The data is now structured, interlinked, and visualized via embedded dashboards and visualizations adapted to each client's needs.
Since developing the solution, Redica Systems has worked with over 90% of the world's leading pharmaceutical and medtech companies, saving hundreds of staff hours every month and reducing compliance risk.
Does GoodData look like the better fit?
Get a demo now and see for yourself. It’s commitment-free.